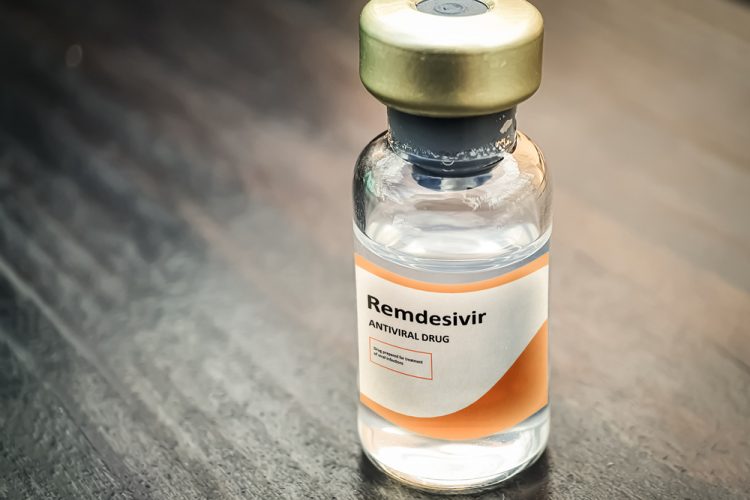
President Donald Trump said on Friday the U.S. Food and Drug Administration (FDA) had granted authorization to Gilead Sciences Inc (GILD.O) for emergency use of its experimental antiviral drug Remdesivir to treat patients with COVID-19.
During a meeting in the Oval Office with the president, Gilead Chief Executive Daniel O’Day called the move an important first step and said the company was donating 1 million vials of the drug to help patients.
What's happening in Tunisia?
Subscribe to our Youtube channel for updates.